Background: Multiple myeloma (MM) is an incurable plasma cell malignancy and genetic abnormalities contribute to disease heterogeneity and outcome. Primary abnormalities, namely recurrent immunoglobulin (Ig) heavy chain translocations and hyperdiploidy, occur early in disease course. Secondary events, such as MYC abnormalities occur upon progression. Earlier studies showed MYC abnormalities detected by FISH or by capture sequencing were independently associated with poor outcome (Walker, et al., BCJ, 2014), while recent studies using WGS did not support this finding (excluding MYC/IGL) (Mikulasova, et al. Haematologica, 2020; Misund, et al. Leukemia, 2020). We hypothesize these discrepancies are due to differences in methods and sensitivities of detection of MYC abnormalities by FISH vs. WGS. Given that MYC abnormalities often display remarkable genomic heterogeneity with numerous gene partners, reduced detection of MYC abnormalities by FISH is not unexpected. This hypothesis is supported by lower frequencies of MYC abnormalities found by FISH (15%) vs. NGS (30-35%) consistent with ~50% false-negative rate of the MYC FISH probe (Smadbeck, et al. BCJ, 2019). To better understand the role of MYC in myeloma disease outcome, we compared the MYC abnormality subtype identified by FISH or NGS vs. MYC gene expression levels and overall survival.
Methods: We performed a retrospective study of newly diagnosed MM patients seen at Mayo Clinic or enrolled in the MMRF CoMMpass trial. For Mayo cases, MYC FISH results (breakapart probe, Abbott) were obtained from the Mayo Clinic Genomics database (N=1342) and mate pair sequencing (MPseq) was performed on 140 cases. For CoMMpass cases, we obtained tumor long-insert whole genome sequencing (WGS), RNA sequencing (RNAseq) for gene expression and clinical outcome data. Overall survival (OS) was defined as time from diagnosis to death from any cause or to last follow up. Survival curves were estimated using Kaplan Meier and compared using the Log-Rank test. Statistical analyses performed using SPSS and JMP with significance determined when P <0.05.
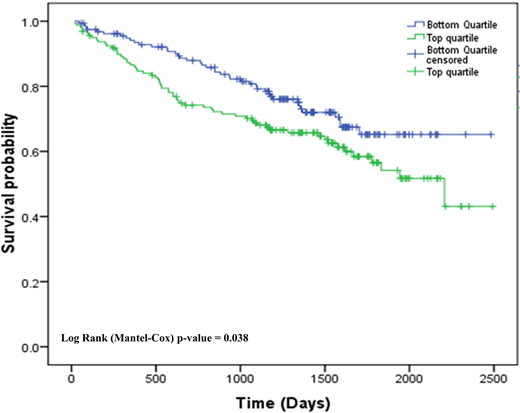
Results: We first evaluated the impact of MYC abnormalities on OS when detected by FISH or NGS. In Mayo cases, OS was significantly shorter in patients with MYC abnormalities compared to patients without MYC abnormalities using FISH (5.3 vs. 8.0 years, P<0.001, N=1342). In contrast, there was no significant difference in OS between patients with or without MYC or abnormalities using MPseq or WGS in both the Mayo and CoMMpass cohorts (Mayo: 6.4 vs. and 6.9 years P=0.78, N=140; CoMMpass: 4.9 vs. and 5.1 years P=0.74, N=546). Since FISH-detected MYC abnormalities were associated with poor outcome, we evaluated differences in the types of MYC abnormalities identified FISH and genome sequencing; 270 of 658 CoMMpass cases had a MYC abnormality and 12 abnormality subgroups were identified. In the Mayo cases, FISH preferentially detected translocations and complex abnormalities and missed insertions with flanking duplicating sequences or terminal tandem duplications (TTD) that occur telomeric to MYC. Since the level of MYC expression should be a consequence of the various genomic abnormalities altering the MYC gene region, we compared MYC expression levels in relation to MYC abnormality subgroups. Highest expression was seen with MYC amplification, followed by Ig abnormalities, non-Ig abnormalities, complex deletion/duplications, proximal deletions, non-Ig insertions, terminal deletions, TTD, trisomy 8, no MYC structural variation, monosomy 8 and cases with MAX mutations had the lowest expression. Abnormalities identified by FISH had higher MYC expression (83.5 TPM) compared to cases predicted to be missed by FISH (63.2 TPM). We tested if high MYC expression, irrespective of MYC structural abnormality, was associated with differences in OS. Boxplot analysis was used to categorize MYC expression in 631 CoMMpass patients as top quartile/high MYC expression (Q4≥ 75 TPM, n=159) and bottom quartile/low MYC expression (Q1≤ 16.5 TPM, n= 158) (see Figure). OS was significantly shorter in patients with high MYC expression compared to patients with low MYC expression (4.6 vs. 5.3 years, P <0.038).
Conclusion: We show that FISH detects only a subset of the MYC abnormalities detected by genome sequencing, and that FISH-detected MYC abnormalities are associated with higher MYC gene expression and decreased survival.
Kumar:Kite Pharma: Consultancy, Research Funding; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; Dr. Reddy's Laboratories: Honoraria; Cellectar: Other; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Novartis: Research Funding; Tenebio: Other, Research Funding; Carsgen: Other, Research Funding; Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Merck: Consultancy, Research Funding; Genecentrix: Consultancy; BMS: Consultancy, Research Funding; Karyopharm: Consultancy; Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Sanofi: Research Funding; MedImmune: Research Funding; Adaptive Biotechnologies: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal